Irb Research Submission Process
Irb Research Submission Process - Irb process duke health institutional review board. What type of submission is this Irb
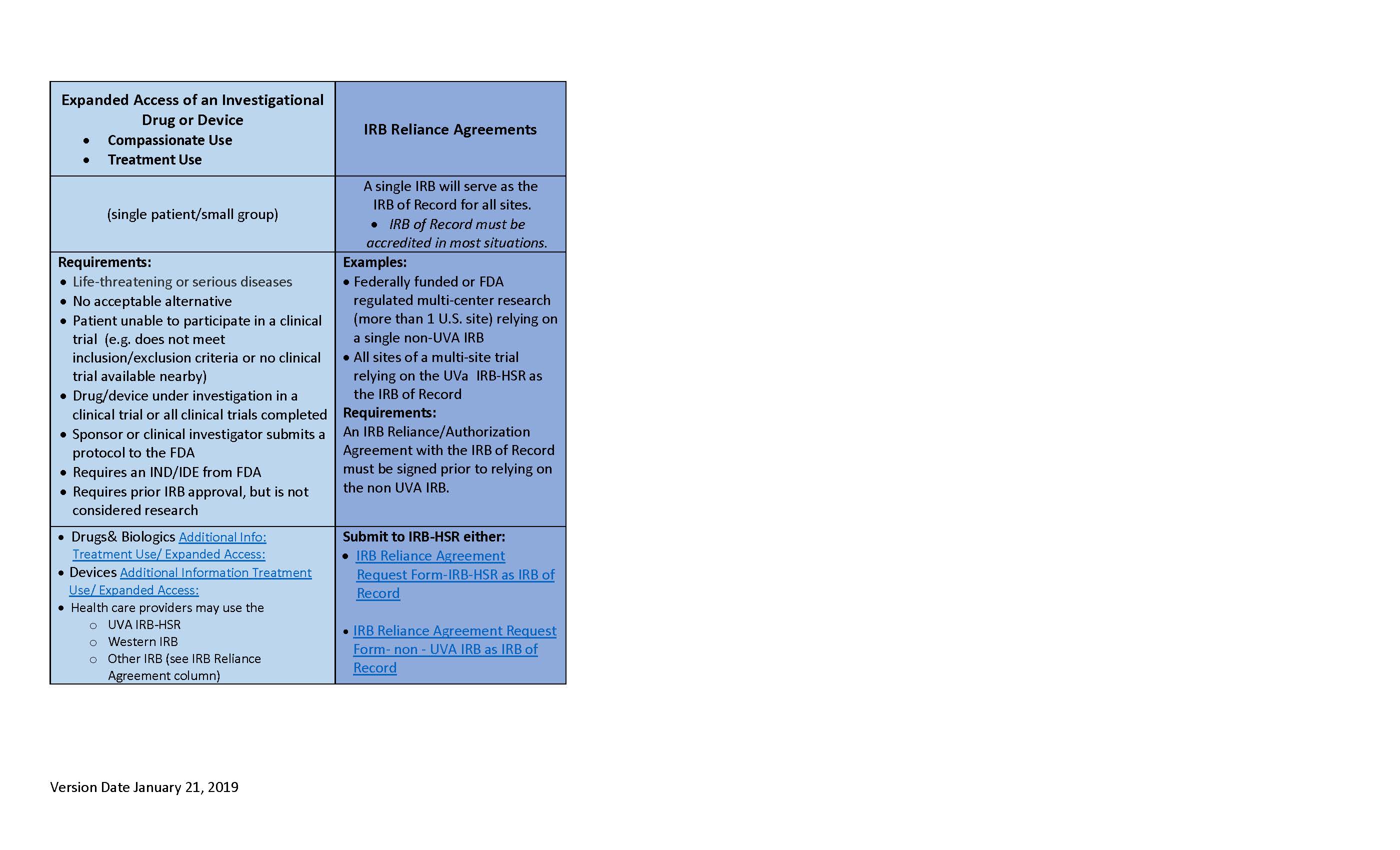
Irb Research Submission Process
Dec 14 2021 nbsp 0183 32 Learn how to register an IRB with OHRP online Update an IRB registration within 90 days after a change in contact person or chairperson and renew IRB registration every 3 years Access FAQs about the IRB registration process IRB Organizations IORGs can register one or more IRB s The Immigration an d Refugee Board of Canada (IRB) is Canada's largest independent administrative tribunal. It is responsible for making well-reasoned decisions on immigration and refugee matters, efficiently, fairly and in accordance with the law.
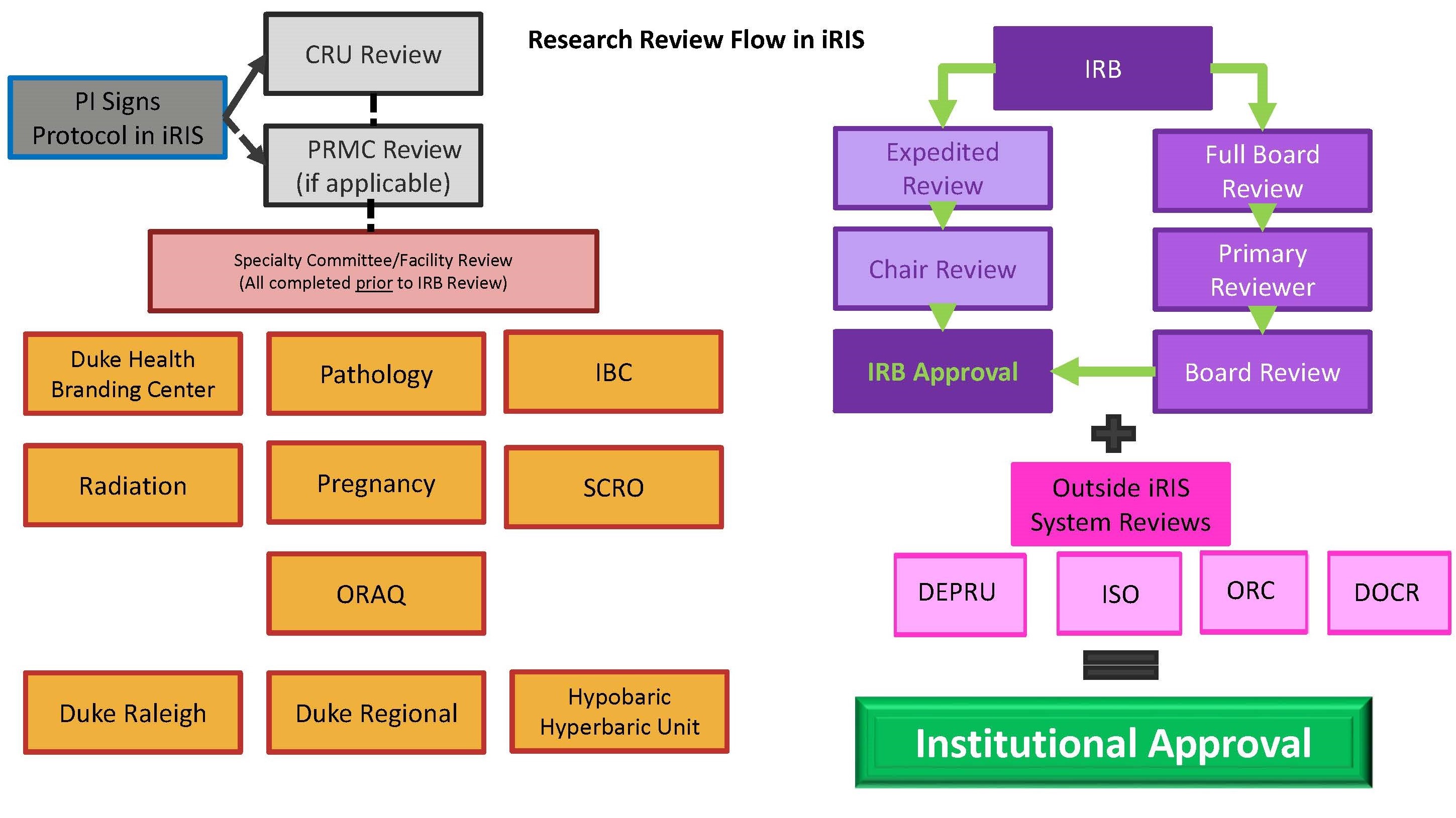
IRB Process Duke Health Institutional Review Board

IRB Approval pdf DocDroid
Irb Research Submission ProcessFeb 24, 2024 · The institutional review board (IRB) is a research ethics committee that reviews and approves human subjects’ research. Most countries use some form of IRB to safeguard ethical conduct of research so that it complies with national and international norms regulations or codes 1 The purpose of the IRB is to assure that appropriate steps are taken to protect the rights and welfare of people participating in a research study
Gallery for Irb Research Submission Process

IRB Review Process Flow Chart Sociology Methods Pinterest

What Type Of Submission Is This
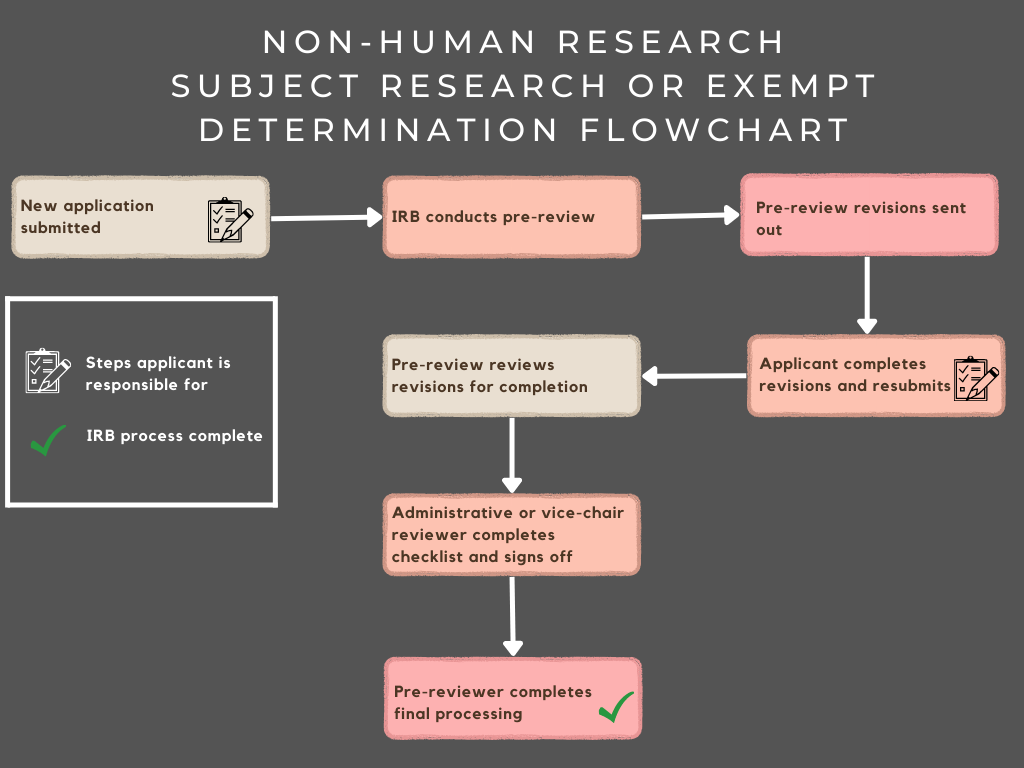
IRB Flow Chart Office Of Undergraduate Research

IRB
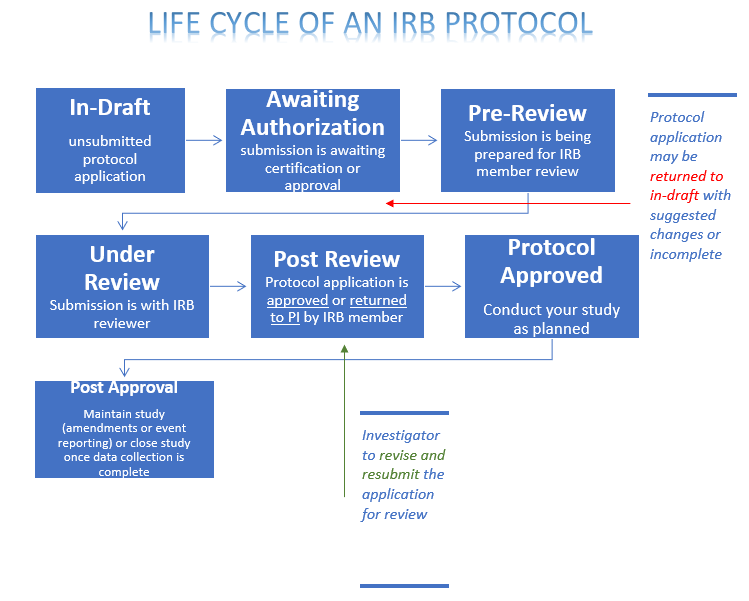
IRB

Institutional Review Board IRB Research In Ethics At CPP Ppt Download

Tarleton State University Ppt Download

Oncology Clinical Trials Ppt Download
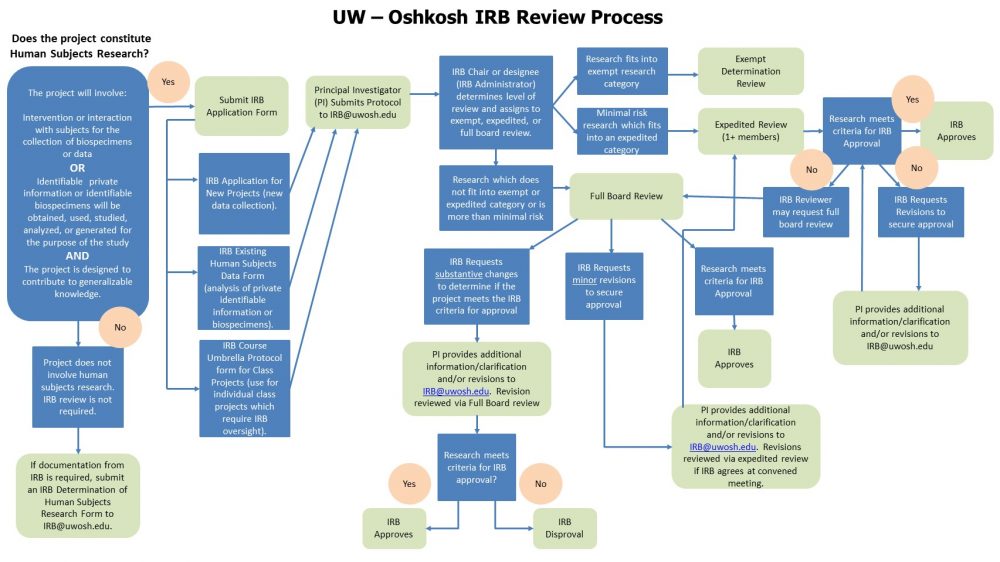
IRB Review Process Flowchart 2022 Office Of Sponsored Programs

Scholars In Medicine 2015 Student Projects IRB Submission Process Ppt